Ni(0)-promoted activation of Csp2–H and Csp2–O bonds†
Abstract
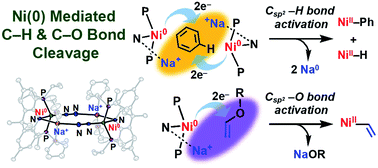
A dinickel(0)–N2 complex, stabilized with a rigid acridane-based PNP pincer ligand, was studied for its ability to activate C(sp2)–H and C(sp2)–O bonds. Stabilized by a Ni–μ–N2–Na+ interaction, it activates C–H bonds of unfunctionalized arenes, affording nickel–aryl and nickel–hydride products. Concomitantly, two sodium cations get reduced to Na(0), which was identified and quantified by several methods. Our experimental results, including product analysis and kinetic measurements, strongly suggest that this C(sp2)–H activation does not follow the typical oxidative addition mechanism occurring at a low-valent single metal centre. Instead, via a bimolecular pathway, two powerfully reducing nickel ions cooperatively activate an arene C–H bond and concomitantly reduce two Lewis acidic alkali metals under ambient conditions. As a novel synthetic protocol, nickel(II)–aryl species were directly synthesized from nickel(II) precursors in benzene or toluene with excess Na under ambient conditions. Furthermore, when the dinickel(0)–N2 complex is accessed via reduction of the nickel(II)–phenyl species, the resulting phenyl anion deprotonates a C–H bond of glyme or 15-crown-5 leading to C–O bond cleavage, which produces vinyl ether. The dinickel(0)–N2 species then cleaves the C(sp2)–O bond of vinyl ether to produce a nickel(II)–vinyl complex. These results may provide a new strategy for the activation of C–H and C–O bonds mediated by a low valent nickel ion supported by a structurally rigidified ligand scaffold.


 Please wait while we load your content...
Please wait while we load your content...