Stereoselective rhodium-catalyzed 2-C–H 1,3-dienylation of indoles: dual functions of the directing group†
Abstract
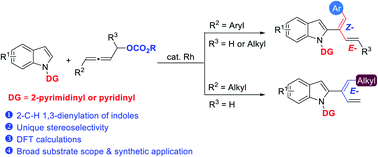
A rhodium-catalyzed intermolecular highly stereoselective 1,3-dienylation at the 2-position of indoles with non-terminal allenyl carbonates has been developed by using 2-pyrimidinyl or pyridinyl as the directing group. The reaction tolerates many functional groups affording the products in decent yields under mild conditions. In addition to C–H bond activation, the directing group also played a vital role in the determination of Z-stereoselectivity for the C–H functionalization reaction with 4-aryl-2,3-allenyl carbonates, which is confirmed by the E-selectivity observed with 4-alkyl-2,3-allenyl carbonates. DFT calculations have been conducted to reveal that π–π stacking involving the directing 2-pyrimidinyl or pyridinyl group is the origin of the observed stereoselectivity. Various synthetic transformations have also been demonstrated.


 Please wait while we load your content...
Please wait while we load your content...