Tandem aza-Heck Suzuki and carbonylation reactions of O-phenyl hydroxamic ethers: complex lactams via carboamination†
Abstract
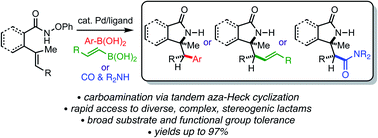
The palladium-catalysed tandem aza-Heck–Suzuki and aza-Heck–carbonylation reactions of O-phenyl hydroxamic ethers are reported. These formal alkene carboamination reactions provide highly versatile access to wide range complex, stereogenic secondary lactams and exhibit outstanding functional group tolerance and high diastereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...