Carbene-catalyzed enantioselective annulation of dinucleophilic hydrazones and bromoenals for access to aryl-dihydropyridazinones and related drugs†
Abstract
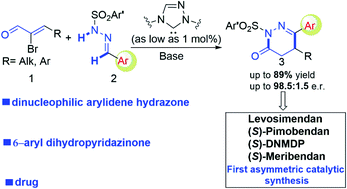
4,5-Dihydropyridazinones bearing an aryl substituent at the C6-position are important motifs in drug molecules. Herein, we developed an efficient protocol to access aryl-dihydropyridazinone molecules via carbene-catalyzed asymmetric annulation between dinucleophilic arylidene hydrazones and bromoenals. Key steps in this reaction include polarity-inversion of aryl aldehyde-derived hydrazones followed by chemo-selective reaction with enal-derived α,β-unsaturated acyl azolium intermediates. The aryl-dihydropyridazinone products accessed by our protocol can be readily transformed into drugs and bioactive molecules.


 Please wait while we load your content...
Please wait while we load your content...