Generation and application of Cu-bound alkyl nitrenes for the catalyst-controlled synthesis of cyclic β-amino acids†
Abstract
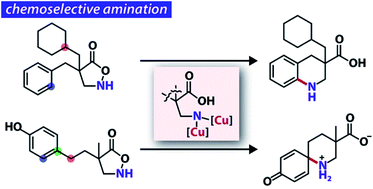
The advent of saturated N-heterocycles as valuable building blocks in medicinal chemistry has led to the development of new methods to construct such nitrogen-containing cyclic frameworks. Despite the apparent strategic clarity, intramolecular C–H aminations with metallonitrenes have only sporadically been explored in this direction because of the intractability of the requisite alkyl nitrenes. Here, we report copper-catalysed intramolecular amination using an alkyl nitrene generated from substituted isoxazolidin-5-ones upon N–O bond cleavage. The copper catalysis exclusively aminates aromatic C(sp2)–H bonds among other potentially reactive groups, offering a solution to the chemoselectivity problem that has been troublesome with rhodium catalysis. A combined experimental and computational study suggested that the active species in the current cyclic β-amino acid synthesis is a dicopper alkyl nitrene, which follows a cyclisation pathway distinct from the analogous alkyl metallonitrene.


 Please wait while we load your content...
Please wait while we load your content...