Scalable synthesis and coupling of quaternary α-arylated amino acids: α-aryl substituents are tolerated in α-helical peptides†
Abstract
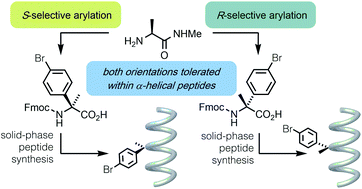
Quaternary amino acids are important tools for the modification and stabilisation of peptide secondary structures. Here we describe a practical and scalable synthesis applicable to quaternary alpha-arylated amino acids (Q4As), and the development of solid-phase synthesis conditions for their incorporation into peptides. Monomeric and dimeric α-helical peptides are synthesised with varying degrees of Q4A substitution and their structures examined using biophysical methods. Both enantiomers of the Q4As are tolerated in folded monomeric and oligomeric α-helical peptides, with the (R)-enantiomer slightly more so than the (S).


 Please wait while we load your content...
Please wait while we load your content...