Prebiotic access to enantioenriched glyceraldehyde mediated by peptides†
Abstract
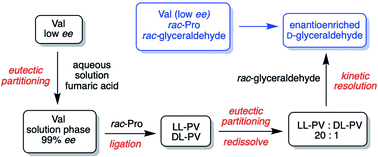
A prebiotically plausible route to enantioenriched glyceraldehyde is reported via a kinetic resolution mediated by peptides. The reaction proceeds via a selective reaction between the L-peptide and the L-sugar producing an Amadori rearrangement byproduct and leaving D-glyceraldehyde in excess. Solubility considerations in the synthesis of proline–valine (pro–val) peptides allow nearly enantiopure pro–val to be formed starting from racemic pro and nearly racemic (10%) ee val. (ee = enantiomeric excess = (|D − L|)/(D + L)) Thus enantioenrichment of glyceraldehyde is achieved in a system with minimal initial chiral bias. This work demonstrates synergy between amino acids and sugars in the emergence of biological homochirality.


 Please wait while we load your content...
Please wait while we load your content...