Unexpected formation of 1,2- and 1,4-bismethoxyl Sc3N@Ih-C80 derivatives via regioselective anion addition: an unambiguous structural identification and mechanism study†
Abstract
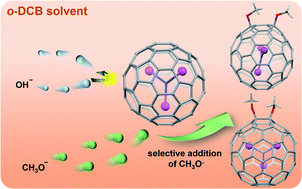
An attempt to achieve heterocyclic cycloadducts of Sc3N@Ih-C80via reaction with Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PhC
O, PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh or PhC
CPh or PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N in the presence of tetrabutylammonium hydroxide (TBAOH) stored in CH3OH led to the formation of the unexpected bismethoxyl adducts of Sc3N@Ih-C80 (1 and 2). Further studies reveal that TBAOH in CH3OH can boost the CH3O− addition efficiently, regardless of the presence of other reagents. Single-crystal X-ray diffraction results firmly assign the molecular structures of 1 and 2 as respective 1,4- and 1,2-bismethoxyl adducts, and reveal unusual relationships between the internal Sc3N cluster and the addition modes, in addition to the unusual packing mode in view of the orientation of the methoxyl groups. Electrochemical results demonstrate smaller electrochemical gaps for 1 and 2, relative to that of Sc3N@Ih-C80, confirming their better electroactive properties. Finally, a plausible reaction mechanism involving anion addition and a radical reaction was proposed, presenting new insights into the highly selective reactions between the methoxyl anion and metallofullerenes. 1 and 2 represent the first examples of methoxyl derivatives of metallofullerenes. This work not only presents a novel and facile strategy for the controllable synthesis of alkoxylated metallofullerene derivatives, but also provides new non-cycloadducts for the potential applications of EMFs.
N in the presence of tetrabutylammonium hydroxide (TBAOH) stored in CH3OH led to the formation of the unexpected bismethoxyl adducts of Sc3N@Ih-C80 (1 and 2). Further studies reveal that TBAOH in CH3OH can boost the CH3O− addition efficiently, regardless of the presence of other reagents. Single-crystal X-ray diffraction results firmly assign the molecular structures of 1 and 2 as respective 1,4- and 1,2-bismethoxyl adducts, and reveal unusual relationships between the internal Sc3N cluster and the addition modes, in addition to the unusual packing mode in view of the orientation of the methoxyl groups. Electrochemical results demonstrate smaller electrochemical gaps for 1 and 2, relative to that of Sc3N@Ih-C80, confirming their better electroactive properties. Finally, a plausible reaction mechanism involving anion addition and a radical reaction was proposed, presenting new insights into the highly selective reactions between the methoxyl anion and metallofullerenes. 1 and 2 represent the first examples of methoxyl derivatives of metallofullerenes. This work not only presents a novel and facile strategy for the controllable synthesis of alkoxylated metallofullerene derivatives, but also provides new non-cycloadducts for the potential applications of EMFs.


 Please wait while we load your content...
Please wait while we load your content...