Effect of charge-transfer enhancement on the efficiency and rotary mechanism of an oxindole-based molecular motor†
Abstract
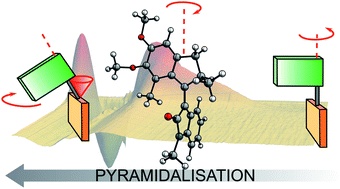
Harvesting energy and converting it into mechanical motion forms the basis for both natural and artificial molecular motors. Overcrowded alkene-based light-driven rotary motors are powered through sequential photochemical and thermal steps. The thermal helix inversion steps are well characterised and can be manipulated through adjustment of the chemical structure, however, the insights into the photochemical isomerisation steps still remain elusive. Here we report a novel oxindole-based molecular motor featuring pronounced electronic push–pull character and a four-fold increase of the photoisomerization quantum yield in comparison to previous motors of its class. A multidisciplinary approach including synthesis, steady-state and transient absorption spectroscopies, and electronic structure modelling was implemented to elucidate the excited state dynamics and rotary mechanism. We conclude that the charge-transfer character of the excited state diminishes the degree of pyramidalisation at the alkene bond during isomerisation, such that the rotational properties of this oxindole-based motor stand in between the precessional motion of fluorene-based molecular motors and the axial motion of biomimetic photoswitches.


 Please wait while we load your content...
Please wait while we load your content...