Catalytic asymmetric addition of thiols to silyl glyoxylates for synthesis of multi-hetero-atom substituted carbon stereocenters†
Abstract
A chiral Lewis acid-catalyzed enantioselective addition of thiols to silyl glyoxylates was developed. The reaction proceeds well with a broad range of thiols and acylsilanes, affording the target tertiary chiral α-silyl–α-sulfydryl alcohols with multi-hetero-atom carbon stereocenters in excellent yields (up to 99%) and enantioselectivities (up to 98% ee). A series of control experiments were conducted to elucidate the reaction mechanism.
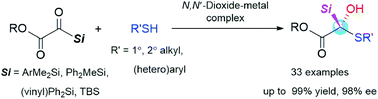


 Please wait while we load your content...
Please wait while we load your content...