Anion mediated, tunable isoguanosine self-assemblies: decoding the conformation influence and solvent effects†‡
Abstract
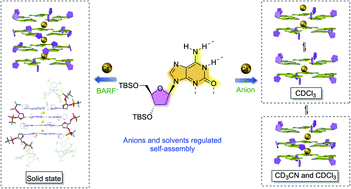
Systematic investigations were performed with various substituted groups at C8 purine and ribose. A series of isoG analogs, C8-phenyl substituted isoG were synthesized and applied for Cs+ coordination. The structural proximity between purine and ribose limited pentaplex formation for C8-phenyl substituted isoG derivatives. Based on this observation, deoxy isoG derivative with modification on ribose (tert-butyldimethylsilyl ether) was applied to assemble with the Cs+ cation. Critical solvent (CDCl3 and CD3CN) and anion (BPh4−, BARF−, and PF6−) effects were revealed, leading to the controllable formation of various stable isoG pentaplexes, including singly charged decamer, doubly charged decamer, and 15-mer, etc. Finally, the X-ray crystal structure of [isoG20Cs3]3+(BARF−)3 was successfully obtained, which is the first example of multiple-layer deoxy isoG binding with the Cs+ cation, providing solid evidence of this new isoG ionophore beyond two-layer sandwich self-assembly.


 Please wait while we load your content...
Please wait while we load your content...