Deaminative meta-C–H alkylation by ruthenium(ii) catalysis†
Abstract
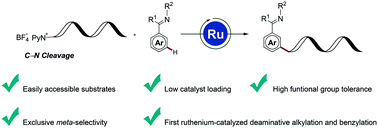
Precise structural modifications of amino acids are of importance to tune biological properties or modify therapeutical capabilities relevant to drug discovery. Herein, we report a ruthenium-catalyzed meta-C–H deaminative alkylation with easily accessible amino acid-derived Katritzky pyridinium salts. Likewise, remote C–H benzylations were accomplished with high levels of chemoselectivity and remarkable functional group tolerance. The meta-C–H activation approach combined with our deaminative strategy represents a rare example of selectively converting C(sp3)–N bonds into C(sp3)–C(sp2) bonds.
- This article is part of the themed collection: 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...