Asymmetric synthesis, structures, and chiroptical properties of helical cycloparaphenylenes†
Abstract
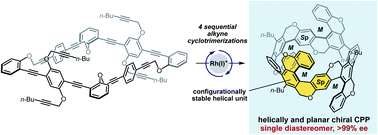
Planar chiral carbon nanorings and nanobelts (CNRs and CNBs), the sidewall segment molecules of chiral-type carbon nanotubes (CNTs), have attracted attention owing to their characteristic chiroptical properties. From the appropriate CNTs, axially or planar chiral CNRs and CNBs have been designed and synthesized, but multiply helical sidewall segments were almost unexplored due to the difficulty in simultaneous control of multiple chiralities. In this article, we have succeeded in the perfectly diastereo- and enantiocontrolled catalytic synthesis of a cycloparaphenylene with four helical and two planar chiralities showing good chiroptical responses as chiral organic molecules. The perfectly stereocontrolled multiply helical structure was confirmed by a single-crystal X-ray diffraction analysis. The experimental and theoretical studies established the importance of the highly symmetric multiply helical structure in the cylindrical axis in obtaining good chiroptical responses.


 Please wait while we load your content...
Please wait while we load your content...