Electrochemically driven stereoselective approach to syn-1,2-diol derivatives from vinylarenes and DMF†
Abstract
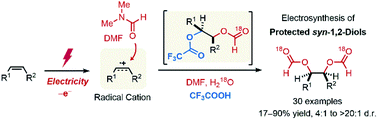
We have developed an electrochemically driven strategy for the stereoselective synthesis of protected syn-1,2-diols from vinylarenes with N,N-dimethylformamide (DMF). The newly developed system obviates the need for transition metal catalysts or external oxidizing agents, thus providing an operationally simple and efficient route to an array of protected syn-1,2-diols in a single step. This reaction proceeds via an electrooxidation of olefin, followed by a nucleophilic attack of DMF. Subsequent oxidation and nucleophilic capture of the generated carbocation with a trifluoroacetate ion is proposed, which gives rise predominantly to a syn-diastereoselectivity upon the second nucleophilic attack of DMF.


 Please wait while we load your content...
Please wait while we load your content...