An insight, at the atomic level, into the polarization effect in controlling the morphology of metal nanoclusters†
Abstract
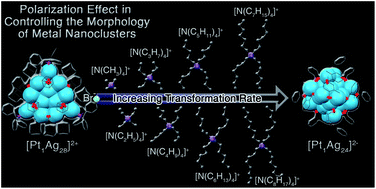
The polarization effect has been a powerful tool in controlling the morphology of metal nanoparticles. However, a precise investigation of the polarization effect has been a challenging pursuit for a long time, and little has been achieved for analysis at the atomic level. Here the atomic-level analysis of the polarization effect in controlling the morphologies of metal nanoclusters is reported. By simply regulating the counterions, the controllable transformation from Pt1Ag28(S-PhMe2)x(S-Adm)18−x(PPh3)4 (x = 0–6, Pt1Ag28-2) to Pt1Ag24(S-PhMe2)18 (Pt1Ag24) with a spherical configuration or to Pt1Ag28(S-Adm)18(PPh3)4 (Pt1Ag28-1) with a tetrahedral configuration has been accomplished. In addition, the spherical or tetrahedral configuration of the clusters could be reversibly transformed by re-regulating the proportion of counterions with opposite charges. More significantly, the configuration transformation rate has been meticulously manipulated by regulating the polarization effect of the ions on the parent nanoclusters. The observations in this paper provide an intriguing nanomodel that enables the polarization effect to be understood at the atomic level.


 Please wait while we load your content...
Please wait while we load your content...