Rhodium-catalyzed cascade reactions of triazoles with organoselenium compounds – a combined experimental and mechanistic study†
Abstract
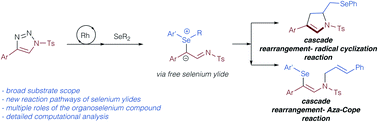
Herein, we report on our studies on the reaction of organoselenium compounds with triazoles under thermal conditions using simple Rh(II) catalysts. These reactions do not provide the product of classic rearrangement reactions. Instead two different cascade reactions were uncovered. While allyl selenides react in a cascade of sigmatropic rearrangement and selenium-mediated radical cyclization reaction to give dihydropyrroles, cinnamyl selenides undergo a double rearrangement reaction cascade involving a final aza-Cope reaction to give the product of 1,3-difunctionalization. Theoretical and experimental studies were conducted to provide an understanding of the reaction mechanism of these cascade reactions. The former provide an important insight into fundamental question on the nature of the ylide intermediate in rearrangement reactions and reveal that organoselenium compounds take up multiple roles in rearrangement reactions and mediate a free ylide reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...