Dual Ag/Co cocatalyst synergism for the highly effective photocatalytic conversion of CO2 by H2O over Al-SrTiO3†
Abstract
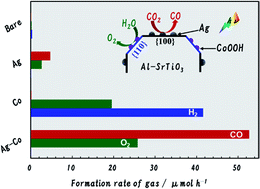
Loading Ag and Co dual cocatalysts on Al-doped SrTiO3 (AgCo/Al-SrTiO3) led to a significantly improved CO-formation rate and extremely high selectivity toward CO evolution (99.8%) using H2O as an electron donor when irradiated with light at wavelengths above 300 nm. Furthermore, the CO-formation rate over AgCo/Al-SrTiO3 (52.7 μmol h−1) was a dozen times higher than that over Ag/Al-SrTiO3 (4.7 μmol h−1). The apparent quantum efficiency for CO evolution over AgCo/Al-SrTiO3 was about 0.03% when photoirradiated at a wavelength at 365 nm, with a CO-evolution selectivity of 98.6% (7.4 μmol h−1). The Ag and Co cocatalysts were found to function as reduction and oxidation sites for promoting the generation of CO and O2, respectively, on the Al-SrTiO3 surface.


 Please wait while we load your content...
Please wait while we load your content...