Heterocyclic group transfer reactions with I(iii) N-HVI reagents: access to N-alkyl(heteroaryl)onium salts via olefin aminolactonization†
Abstract
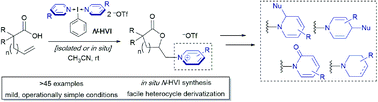
Pyridinium and related N-alkyl(heteroaryl)onium salts are versatile synthetic intermediates in organic chemistry, with applications ranging from ring functionalizations to provide diverse piperidine scaffolds to their recent emergence as radical precursors in deaminative cross couplings. Despite their ever-expanding applications, methods for their synthesis have seen little innovation, continuing to rely on a limited set of decades old transformations and a limited subset of coupling partners. Herein, we leverage (bis)cationic nitrogen-ligated I(III) hypervalent iodine reagents, or N-HVIs, as “heterocyclic group transfer reagents” to provide access to a broad scope of N-alkyl(heteroaryl)onium salts via the aminolactonization of alkenoic acids, the first example of engaging an olefin to directly generate these salts. The reactions proceed in excellent yields, under mild conditions, and are capable of incorporating a broad scope of sterically and electronically diverse aromatic heterocycles. The N-HVI reagents can be generated in situ, the products isolated via simple trituration, and subsequent derivatizations demonstrate the power of this platform for diversity-oriented synthesis of 6-membered nitrogen heterocycles.
- This article is part of the themed collection: Most popular 2021 organic chemistry articles, 2021


 Please wait while we load your content...
Please wait while we load your content...