The transformations of a methylene-bridged bis-triazolium salt: a mesoionic carbene based metallocage and analogues of TCNE and NacNac†‡
Abstract
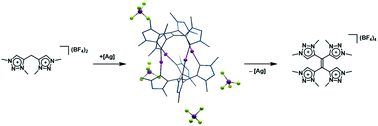
Unusual and unexpected chemical transformations often provide access to completely new types of functional molecules. We report here the synthesis of a methylene-bridged bis-triazolium salt designed as a precursor for a new bis-mesoionic carbene (MIC) ligand. The direct metalation with silver oxide led to the isolation and crystallographic characterization of a cationic tetranuclear octacarbene–silver(I) complex. During metalation the formal bis-MIC precursor undergoes significant structural changes and chemical transformations. A combined synthetic, crystallographic and (spectro-)electrochemical approach is used to elucidate the mechanistic pathway: starting from the methylene-bridged bis-triazolium salt a single deprotonation leads to a NacNac analogue, which is followed by a redox-induced radical dimerization reaction, generating a new tetra-MIC ligand coordinated to silver(I) central atoms. Decomplexation led to the isolation of the corresponding tetratriazoliumethylene, a profoundly electron-poor alkene, which is an analogue of TCNE.


 Please wait while we load your content...
Please wait while we load your content...