Self-healing and shape memory functions exhibited by supramolecular liquid-crystalline networks formed by combination of hydrogen bonding interactions and coordination bonding†
Abstract
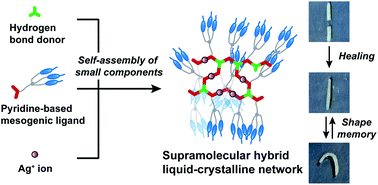
We here report a new approach to develop self-healing shape memory supramolecular liquid-crystalline (LC) networks through self-assembly of molecular building blocks via combination of hydrogen bonding and coordination bonding. We have designed and synthesized supramolecular LC polymers and networks based on the complexation of a forklike mesogenic ligand with Ag+ ions and carboxylic acids. Unidirectionally aligned fibers and free-standing films forming layered LC nanostructures have been obtained for the supramolecular LC networks. We have found that hybrid supramolecular LC networks formed through metal–ligand interactions and hydrogen bonding exhibit both self-healing properties and shape memory functions, while hydrogen-bonded LC networks only show self-healing properties. The combination of hydrogen bonds and metal–ligand interactions allows the tuning of intermolecular interactions and self-assembled structures, leading to the formation of the dynamic supramolecular LC materials. The new material design presented here has potential for the development of smart LC materials and functional LC membranes with tunable responsiveness.


 Please wait while we load your content...
Please wait while we load your content...