Chemoproteomic profiling of kinases in live cells using electrophilic sulfonyl triazole probes†
Abstract
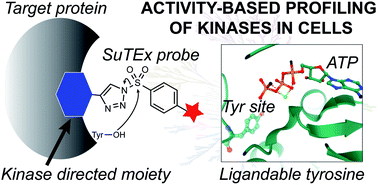
Sulfonyl-triazoles are a new class of electrophiles that mediate covalent reaction with tyrosine residues on proteins through sulfur-triazole exchange (SuTEx) chemistry. Recent studies demonstrate the broad utility and tunability of SuTEx chemistry for chemical proteomics and protein ligand discovery. Here, we present a strategy for mapping protein interaction networks of structurally complex binding elements using functionalized SuTEx probes. We show that the triazole leaving group (LG) can serve as a releasable linker for embedding hydrophobic fragments to direct molecular recognition while permitting efficient proteome-wide identification of binding sites in live cells. We synthesized a series of SuTEx probes functionalized with a lipid kinase fragment binder for discovery of ligandable tyrosines residing in catalytic and regulatory domains of protein and metabolic kinases in live cells. We performed competition studies with kinase inhibitors and substrates to demonstrate that probe binding is occurring in an activity-dependent manner. Our functional studies led to discovery of probe-modified sites within the C2 domain that were important for downregulation of protein kinase C-alpha in response to phorbol ester activation. Our proof of concept studies highlight the triazole LG of SuTEx probes as a traceless linker for locating protein binding sites targeted by complex recognition elements in live cells.


 Please wait while we load your content...
Please wait while we load your content...