Iridium-promoted deoxyglycoside synthesis: stereoselectivity and mechanistic insight†
Abstract
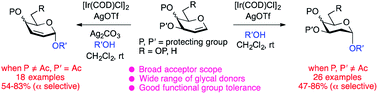
Herein, we devised a method for stereoselective O-glycosylation using an Ir(I)-catalyst which enables both hydroalkoxylation and nucleophilic substitution of glycals with varying substituents at the C3 position. In this transformation, 2-deoxy-α-O-glycosides were acquired when glycals equipped with a notoriously poor leaving group at C3 were used; in contrast 2,3-unsaturated-α-O-glycosides were produced from glycals that bear a good leaving group at C3. Mechanistic studies indicate that both reactions proceed via the directing mechanism, through which the acceptor coordinates to the Ir(I) metal in the α-face-coordinated Ir(I)-glycal π-complex and then attacks the glycal that contains the O-glycosidic bond in a syn-addition manner. This protocol exhibits good functional group tolerance and is exemplified with the preparation of a library of oligosaccharides in moderate to high yields and with excellent stereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...