A pyrone remodeling strategy to access diverse heterocycles: application to the synthesis of fascaplysin natural products†
Abstract
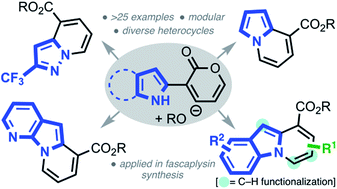
The synthesis of diverse N-fused heterocycles, including the pyrido[1,2-a]indole scaffold, using an efficient pyrone remodeling strategy is described. The pyrido[1,2-a]indole core was demonstrated to be a versatile scaffold that can be site-selectively functionalized. The utility of this novel annulation strategy was showcased in a concise formal synthesis of three fascaplysin congeners.


 Please wait while we load your content...
Please wait while we load your content...