Sterically constrained tricyclic phosphine: redox behaviour, reductive and oxidative cleavage of P–C bonds, generation of a dilithium phosphaindole as a promising synthon in phosphine chemistry†‡
Abstract
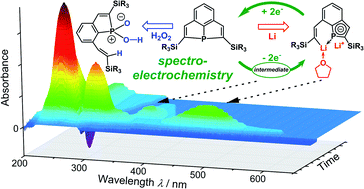
The redox behaviour of sterically constrained tricyclic phosphine 3a was investigated by spectroelectrochemistry. The data suggested a highly negative reduction potential with the reversible formation of a dianionic species. Accordingly, 3a reacted with two equivalents of Li/naphthalene by reductive cleavage of a P–C bond of one of the PC4 heterocycles. The resulting dilithium compound 5 represents a phosphaindole derivative with annulated aromatic C6 and PC4 rings. It is an interesting starting material for the synthesis of new heterocyclic molecules, as was shown by treatment with Me2SiCl2 and PhPCl2. The structures of the products (6 and 7) formally reflect ring expansion by insertion of silylen or phosphinidene fragments into a P–C bond of 3a. Treatment of 3a with H2O2 did not result in the usually observed transfer of a single O atom to phosphorus, but oxidative cleavage of a strained PC4 ring afforded a bicyclic phosphinic acid, R2PO2H.


 Please wait while we load your content...
Please wait while we load your content...