A 2,2′-diphosphinotolane as a versatile precursor for the synthesis of P-ylidic mesoionic carbenes via reversible C–P bond formation†
Abstract
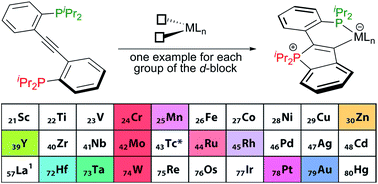
A metal-templated synthetic route to cyclic (aryl)(ylidic) mesoionic carbenes (CArY-MICs) featuring an endocyclic P-ylide is presented. This approach, which requires metal templates with two cis-positioned open coordination sites, is based on the controlled cyclisation of a P,P′-diisopropyl-substituted 2,2′-diphosphinotolane (1) and leads to chelate complexes coordinated by a phosphine donor and the CArY-MIC carbon atom. The C–P bond formation involved in the former partial cyclisation of 1 proceeds under mild conditions and was shown to be applicable all over the d-block. In the presence of a third fac-positioned open coordination site, the P–C bond formation was found to be reversible, as shown for a series of molybdenum complexes. DFT modelling studies are in line with an interpretation of the target compounds as CArY-MICs.


 Please wait while we load your content...
Please wait while we load your content...