The central role of the metal ion for photoactivity: Zn– vs. Ni–Mabiq†
Abstract
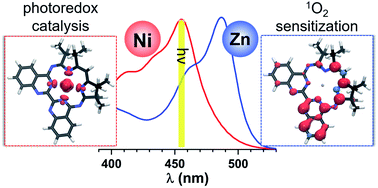
Photoredox catalysts are integral components of artificial photosystems, and have recently emerged as powerful tools for catalysing numerous organic reactions. However, the development of inexpensive and efficient earth-abundant photoredox catalysts remains a challenge. We here present the photochemical and photophysical properties of a Ni–Mabiq catalyst ([NiII(Mabiq)]OTf (1); Mabiq = 2-4:6-8-bis(3,3,4,4-tetramethyldihydropyrrolo)-10-15-(2,2-biquinazolino)-[15]-1,3,5,8,10,14-hexaene1,3,7,9,11,14-N6)—and of a Zn-containing analogue ([ZnII(Mabiq)OTf] (2))—using steady state and time resolved optical spectroscopy, time-dependent density functional theory (TDDFT) calculations, and reactivity studies. The Ni and Zn complexes exhibit similar absorption spectra, but markedly different photochemical properties. These differences arise because the excited states of 2 are ligand-localized, whereas metal-centered states account for the photoactivity of 1. The distinct properties of the Ni and Zn complexes are manifest in their behavior in the photo-driven aza-Henry reaction and oxidative coupling of methoxybenzylamine.


 Please wait while we load your content...
Please wait while we load your content...