Mangana(iii/iv)electro-catalyzed C(sp3)–H azidation†
Abstract
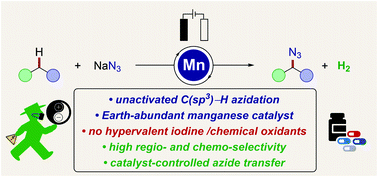
Manganaelectro-catalyzed azidation of otherwise inert C(sp3)–H bonds was accomplished using most user-friendly sodium azide as the nitrogen-source. The operationally simple, resource-economic C–H azidation strategy was characterized by mild reaction conditions, no directing group, traceless electrons as the sole redox-reagent, Earth-abundant manganese as the catalyst, high functional-group compatibility and high chemoselectivity, setting the stage for late-stage azidation of bioactive compounds. Detailed mechanistic studies by experiment, spectrophotometry and cyclic voltammetry provided strong support for metal-catalyzed aliphatic radical formation, along with subsequent azidyl radical transfer within a manganese(III/IV) manifold.


 Please wait while we load your content...
Please wait while we load your content...