Discovery of rare sulfated N-unsubstituted glucosamine based heparan sulfate analogs selectively activating chemokines†
Abstract
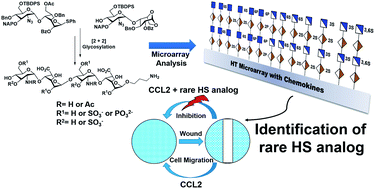
Achieving selective inhibition of chemokines with structurally well-defined heparan sulfate (HS) oligosaccharides can provide important insights into cancer cell migration and metastasis. However, HS is highly heterogeneous in chemical composition, which limits its therapeutic use. Here, we report the rational design and synthesis of N-unsubstituted (NU) and N-acetylated (NA) heparan sulfate tetrasaccharides that selectively inhibit structurally homologous chemokines. HS analogs were produced by divergent synthesis, where fully protected HS tetrasaccharide precursor was subjected to selective deprotection and regioselectively O-sulfated, and O-phosphorylated to obtain 13 novel HS tetrasaccharides. HS microarray and SPR analysis with a wide range of chemokines revealed the structural significance of sulfation patterns and NU domain in chemokine activities for the first time. Particularly, HT-3,6S-NH revealed selective recognition by CCL2 chemokine. Further systematic interrogation of the role of HT-3,6S-NH in cancer demonstrated an effective blockade of CCL2 and its receptor CCR2 interactions, thereby impairing cancer cell proliferation, migration and invasion, a step towards designing novel drug molecules.


 Please wait while we load your content...
Please wait while we load your content...