Three-component three-bond forming cascade via palladium photoredox catalysis†
Abstract
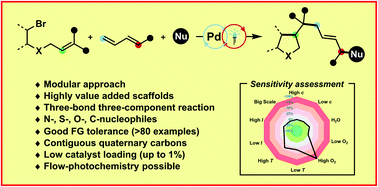
A highly modular radical cascade strategy based upon radical cyclisation/allylic substitution sequence between alkyl/aryl bromides, 1,3-dienes and nucleophiles ranging from sulfinates to amines, phenols and 1,3-dicarbonyls is described (>80 examples). Palladium phosphine complexes – which merge properties of photo- and cross coupling-catalysts – allow to forge three bonds with complete 1,4-selectivity and stereocontrol, delivering highly value added carbocyclic and heterocyclic motifs that can feature – inter alia – vicinal quaternary centers, free protic groups, gem-difluoro motifs and strained rings. Furthermore, a flow chemistry approach was for the first time applied in palladium–photocatalysed endeavors involving radicals.
- This article is part of the themed collections: Most popular 2021 catalysis articles, 2021 and Materials & Energy in Chemical Science - most popular articles 2021


 Please wait while we load your content...
Please wait while we load your content...