Tuning phenoxyl-substituted diketopyrrolopyrroles from quinoidal to biradical ground states through (hetero-)aromatic linkers†
Abstract
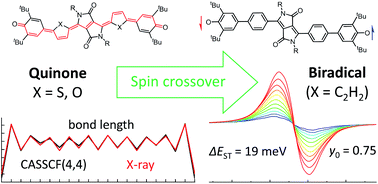
Strongly fluorescent halochromic 2,6-di-tert-butyl-phenol-functionalised phenyl-, thienyl- and furyl-substituted diketopyrrolopyrrole (DPP) dyes were deprotonated and oxidised to give either phenylene-linked DPP1˙˙ biradical (y0 = 0.75) with a singlet open shell ground state and a thermally populated triplet state (ΔEST = 19 meV; 1.8 kJ mol−1; 0.43 kcal mol−1) or thienylene/furylene-linked DPP2q and DPP3q compounds with closed shell quinoidal ground states. Accordingly, we identified the aromaticity of the conjugated (hetero-)aromatic bridge to be key for modulating the electronic character of these biradicaloid compounds and achieved a spin crossover from closed shell quinones DPP2q and DPP3q to open shell biradical DPP1˙˙ as confirmed by optical and magnetic spectroscopic studies (UV/vis/NIR, NMR, EPR) as well as computational investigations (spin-flip TD-DFT calculations in combination with CASSCF(4,4) and harmonic oscillator model of aromaticity (HOMA) analysis). Spectroelectrochemical studies and comproportionation experiments further prove the reversible formation of mixed-valent radical anions for the DPP2q and DPP3q quinoidal compounds with absorption bands edging into the NIR spectral region.


 Please wait while we load your content...
Please wait while we load your content...