The site-selectivity and mechanism of Pd-catalyzed C(sp2)–H arylation of simple arenes†
Abstract
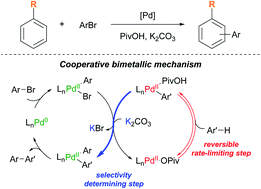
Control over site-selectivity is a critical challenge for practical application of catalytic C–H functionalization reactions in organic synthesis. Despite the seminal breakthrough of the Pd-catalyzed C(sp2)–H arylation of simple arenes via a concerted metalation–deprotonation (CMD) pathway in 2006, understanding the site-selectivity of the reaction still remains elusive. Here, we have comprehensively investigated the scope, site-selectivity, and mechanism of the Pd-catalyzed direct C–H arylation reaction of simple arenes. Counterintuitively, electron-rich arenes preferably undergo meta-arylation without the need for a specifically designed directing group, whereas electron-deficient arenes bearing fluoro or cyano groups exhibit high ortho-selectivity and electron-deficient arenes bearing bulky electron-withdrawing groups favor the meta-product. Comprehensive mechanistic investigations through a combination of kinetic measurements and stoichiometric experiments using arylpalladium complexes have revealed that the Pd-based catalytic system works via a cooperative bimetallic mechanism, not the originally proposed monometallic CMD mechanism, regardless of the presence of a strongly coordinating L-type ligand. Notably, the transmetalation step, which is influenced by a potassium cation, is suggested as the selectivity-determining step.
- This article is part of the themed collection: Celebrating the 75th Anniversary of the Korean Chemical Society (KCS)


 Please wait while we load your content...
Please wait while we load your content...