Born–Oppenheimer approximation in optical cavities: from success to breakdown†
Abstract
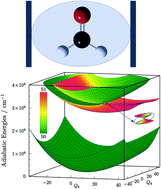
The coupling of a molecule and a cavity induces nonadiabaticity in the molecule which makes the description of its dynamics complicated. For polyatomic molecules, reduced-dimensional models and the use of the Born–Oppenheimer approximation (BOA) may remedy the situation. It is demonstrated that contrary to expectation, BOA may even fail in a one-dimensional model and is generally expected to fail in two- or more-dimensional models due to the appearance of conical intersections induced by the cavity.
- This article is part of the themed collections: Editor’s Choice – Graeme Day and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...