Optimal water concentration for aqueous Li+ intercalation in vanadyl phosphate†
Abstract
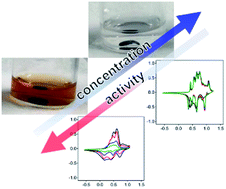
Development of high-performance aqueous batteries is an important goal for energy sustainability owing to their environmental benignity and low fabrication costs. Although a layered vanadyl phosphate is one of the most-studied host materials for intercalation electrodes with organic electrolytes, little attention has been paid to its use in aqueous Li+ systems because of its excessive dissolution in water. Herein, by controlling the water concentration, we demonstrate the stable operation of a layered vanadyl phosphate electrode in an aqueous Li+ electrolyte. The combination of experimental analyses and density functional theory calculations reveals that reversible (de)lithiation occurs between dehydrated phases, which can only exist in an optimal water concentration.
- This article is part of the themed collection: Editor’s Choice: Zaiping Guo


 Please wait while we load your content...
Please wait while we load your content...