Visible-light-driven palladium-catalyzed Dowd–Beckwith ring expansion/C–C bond formation cascade†
Abstract
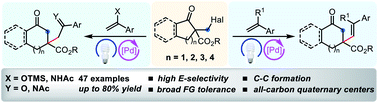
A visible-light-induced palladium-catalyzed Dowd–Beckwith ring expansion/C–C bond formation cascade is described. A range of six to nine-membered β-alkenylated cyclic ketones possessing a quaternary carbon center were accessed under mild conditions. Besides styrenes, the electron-rich alkenes such as silyl enol ethers and enamides were also compatible, providing the desired β-alkylated cyclic ketones in moderate to good yields.


 Please wait while we load your content...
Please wait while we load your content...