Cooperative activating effects of metal ion and Brønsted acid on a metal oxo species†
Abstract
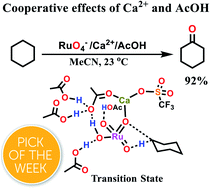
Metal oxo (M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) complexes are common oxidants in chemical and biological systems. The use of Lewis acids to activate metal oxo species has attracted great interest in recent years, especially after the discovery of the CaMn4O5 cluster in the oxygen-evolving centre of photosystem II. Strong Lewis acids such as Sc3+ and BF3, as well as strong Brønsted acids such as H2SO4 and CF3SO3H, are commonly used to activate metal oxo species. In this work, we demonstrate that relatively weak Lewis acids such as Ca2+ and other group 2 metal ions, as well as weak Brønsted acids such as CH3CO2H, can readily activate the stable RuO4− complex towards the oxidation of alkanes. Notably, the use of Ca2+ and CH3CO2H together produces a remarkable cooperative effect on RuO4−, resulting in a much more efficient oxidant. DFT calculations show that Ca2+ and CH3CO2H can bind to two oxo ligands to form a chelate ring. This results in substantial lowering of the barrier for hydrogen atom abstraction from cyclohexane.
O) complexes are common oxidants in chemical and biological systems. The use of Lewis acids to activate metal oxo species has attracted great interest in recent years, especially after the discovery of the CaMn4O5 cluster in the oxygen-evolving centre of photosystem II. Strong Lewis acids such as Sc3+ and BF3, as well as strong Brønsted acids such as H2SO4 and CF3SO3H, are commonly used to activate metal oxo species. In this work, we demonstrate that relatively weak Lewis acids such as Ca2+ and other group 2 metal ions, as well as weak Brønsted acids such as CH3CO2H, can readily activate the stable RuO4− complex towards the oxidation of alkanes. Notably, the use of Ca2+ and CH3CO2H together produces a remarkable cooperative effect on RuO4−, resulting in a much more efficient oxidant. DFT calculations show that Ca2+ and CH3CO2H can bind to two oxo ligands to form a chelate ring. This results in substantial lowering of the barrier for hydrogen atom abstraction from cyclohexane.
- This article is part of the themed collection: 2020 ChemSci Pick of the Week Collection


 Please wait while we load your content...
Please wait while we load your content...