Palladium catalyzed synthesis of indolizines via the carbonylative coupling of bromopyridines, imines and alkynes†
Abstract
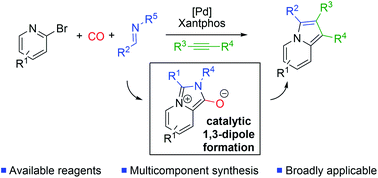
We report herein the development of a palladium-catalyzed, multicomponent synthesis of indolizines. The reaction proceeds via the carbonylative formation of a high energy, mesoionic pyridine-based 1,3-dipole, which can undergo spontaneous cycloaddition with alkynes. Overall, this provides a route to prepare indolizines in a modular fashion from combinations of commercially available or easily generated reagents: 2-bromopyridines, imines and alkynes.


 Please wait while we load your content...
Please wait while we load your content...