Supramolecular adducts between macrocyclic Gd(iii) complexes and polyaromatic systems: a route to enhance the relaxivity through the formation of hydrophobic interactions†
Abstract
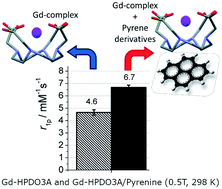
The set-up of reversible binding interactions between the hydrophobic region of macrocyclic GBCAs (Gadolinium Based Contrast Agents) and SO3−/OH containing pyrene derivatives provides new insights for pursuing relaxivity enhancements of this class of MRI contrast agents. The strong binding affinity allows attaining relaxation enhancements up to 50% at pyrene/GBCA ratios of 3 : 1. High resolution NMR spectra of the Yb-HPDO3A/pyrene system fully support the formation of a supramolecular adduct based on the set-up of hydrophobic interactions. The relaxation enhancement may be accounted for in terms of the increase of the molecular reorientation time (τR) and the number of second sphere water molecules. This effect is maintained in blood serum and in vivo, as shown by the enhancement of contrast in T1w-MR images obtained by simultaneous injection of GBCA and pyrene derivatives in mice.


 Please wait while we load your content...
Please wait while we load your content...