Lithium cuprate, a multifunctional material for NO selective catalytic reduction by CO with subsequent carbon oxide capture at moderate temperatures†
Abstract
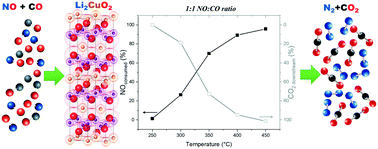
Li2CuO2 was evaluated as a possible catalyst for the NO selective catalytic reduction (NO SCR) by CO. The initial idea was supported by ab initio thermodynamic analysis. This analysis showed that NO SCR was favored by using Li2CuO2 as a catalyst, based on the calculated heat of reaction and Gibbs free energy. The NO SCR reaction was evaluated under dynamic, isothermal and cyclic conditions using different NO : CO ratios (2 : 1, 1 : 1 and 1 : 2). The dynamic and isothermal experiments showed that Li2CuO2 does reduce NO in the presence of CO to reach higher conversions (>90%) between 400 and 450 °C. However, it must be mentioned that the catalytic process was reduced once some of the produced CO2 was captured on the ceramic. At that point, Li2CuO2 acts as a bifunctional material, catalyzing the NO SCR reaction and capturing some of the produced CO2. Moreover, cyclic experiments evidenced high NO reduction. Finally, a different set of experiments showed that this catalytic reduction performed differently if NO and CO were previously fed. When lithium cuprate is previously carbonated (initial CO feed), the catalytic reaction does not complete. In contrast, when there was a previous nitrate formation (initial NO feed), the reaction reached 100% efficiency.


 Please wait while we load your content...
Please wait while we load your content...