Renewable dimethyl carbonate for tertiary amine quaternisation: kinetic measurements and process optimisation
Abstract
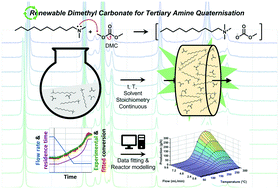
Quaternary ammonium salts (QAS) are an important part of the increasing surfactant market. Conventional production processes employ toxic alkyl halides in a Menshutkin reaction with a tertiary amine (DMDA). Dimethyl carbonate (DMC) can provide a renewable route, while also leading to more benign, and non-corrosive products. This work aims to use linear ramp-flow in a plug flow reactor (PFR), combined with in-line 1H NMR spectroscopy to determine reaction kinetics. These kinetics will be used to further optimise the production process with a computational model. Solvent effects were first studied in a batch reactor. Methanol (MeOH) was found most suitable as a solvent. Subsequently, the reaction kinetics were measured in a PFR set-up. The used ramp-flow was successfully validated with data from batch and steady-state experiments. Arrhenius parameters were determined with the ramp-flow method, which proved to be an accurate and efficient technique. The kinetic data was implemented in a computational model. After validation of the model with experimental data, it was employed to extrapolate this data and optimise the reaction. The optimum QAS productivity was predicted at 122 kg h−1 L−1, obtained at 270 °C, 0.25 min residence time, and a molar ratio of 1 : 2.5 : 10 (DMDA : DMC : MeOH). These conditions would provide significant intensification of the QAS production processes.


 Please wait while we load your content...
Please wait while we load your content...