High-efficiency catalytic reduction of residual oxygen for purification of carbon dioxide streams from high-pressure oxy-combustion systems†
Abstract
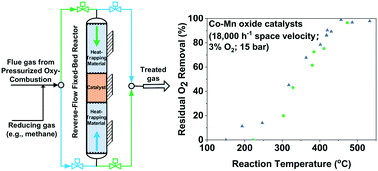
Pressurized oxy-combustion is a promising technology for carbon capture, utilization, and storage. For the captured CO2 to be used for enhanced oil recovery or stored in geological formations, flue gas impurities, including residual O2 in the CO2 stream, must be purified to meet the purity specifications. A catalytic approach to reducing residual O2 with CH4 was investigated in this study. Five CoMn- and Cu-based catalysts were synthesized or acquired, and a reverse-flow fixed-bed reactor was used to assess their performance for O2 removal from a simulated oxy-combustion flue gas at 15 bar. The impacts of the operating parameters on O2 removal, such as temperature, gas hourly space velocity, O2/CH4 ratio, and gas pressure, were investigated. Among the tested catalysts, the two CoMn catalysts were superior in both activity and selectivity, with the reaction lighting off at about 350 °C and achieving 99% O2 removal at about 500 °C. The kinetics of the catalytic reaction is discussed, and the Mars–van Krevelen redox mechanism is deemed valid for describing the reaction pathway for the top-performing CoMn catalysts. The catalytic reaction was determined to be first order in CH4 and zero order in O2 under the test conditions.


 Please wait while we load your content...
Please wait while we load your content...