Enone-promoted decarboxylation of trans-4-hydroxy-l-proline in flow: a side-by-side comparison to batch†
Abstract
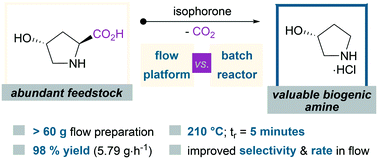
An efficient and scalable enone-promoted method for the decarboxylation of trans-4-hydroxy-proline has been developed in flow to provide access to (R)-pyrrolidin-3-ol hydrochloride using biomass-derived isophorone. The advantages of deploying flow in this setting were reinforced by direct comparsion to batch. This method lays a foundation for fossil fuel and ammonia free alternative access to amines on scales relevant to manufacturing.


 Please wait while we load your content...
Please wait while we load your content...