Efficient Ni-based catalysts for the hydrotreatment of lignin dimer model compounds to cycloalkanes/cycloalkanols†
Abstract
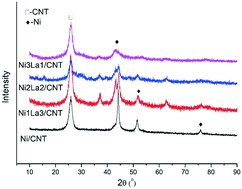
The noble-metal catalytic cleavage of ether bonds in lignin to obtain aromatic chemicals has achieved great success, and the development of a low-cost efficient catalyst is crucial. Herein, NixLay/CNT was designed for the hydrogenolysis of benzyl phenyl ether (BPE), diphenyl ether (DPE) and 2-phenethyl phenyl ether (PPE) under a relatively mild condition. Owing to the synergistic effect of Ni with La, the Ni–La catalyst was especially active. Moreover, not only could the Ni–La catalyst fracture the C–O bond, but it could also transform aromatic rings to produce cycloalkanes. Physicochemical characterizations were carried out by means of XRD, TEM, H2-TPR, NH3-TPD, pyridine-IR and XPS analyses. Based on the optimal reaction condition (240 °C, 4 h, 2.0 MPa H2), various model compounds could also be effectively hydrotreated to produce corresponding products. A mechanistic study revealed that cyclohexanol and methylcyclohexane were major products for the transfer cleavage of BPE. Furthermore, it has been illustrated that aryl groups played a significant role in the hydrogenation of phenol from the competitive catalytic hydrogenation reaction of phenol. This study opened up the possibility of the valorization of lignin using a rare earth metal as the co-catalyst for the selective cleavage of lignin model compounds to value-added chemicals.


 Please wait while we load your content...
Please wait while we load your content...