Design, synthesis, docking study and anticancer evaluation of new trimethoxyphenyl pyridine derivatives as tubulin inhibitors and apoptosis inducers†
Abstract
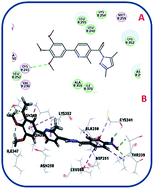
Microtubules have become an appealing target for anticancer drug development including mainly colchicine binding site inhibitors (CBSIs). A new series of novel trimethoxypyridine derivatives were designed and synthesized as tubulin targeting agents. In vitro anti-proliferative activities of the tested compounds compared to colchicine against hepatocellular carcinoma (HepG-2), colorectal carcinoma (HCT-116), and breast cancer (MCF-7) was carried out. Most of compounds showed significant cytotoxic activities. Compounds Vb, Vc, Vf, Vj and VI showed superior anti-proliferative activities to colchicine. Where compound VI showed IC50 values of 4.83, 3.25 and 6.11 μM compared to colchicine (7.40, 9.32, 10.41 μM) against HCT 116, HepG-2 and MCF-7, respectively. The enzymatic activity against tubulin enzyme was carried out for the compounds that showed high anti-proliferative activity. Also, compound VI exhibited the highest tubulin polymerization inhibitory effect with an IC50 value of 8.92 nM compared to colchicine (IC50 value = 9.85 nM). Compounds Vb, Vc, Vf, Vj, & VIIIb showed promising activities with IC50 values of 22.41, 17.64, 20.39, 10.75, 31.86 nM, respectively. Cell cycle and apoptosis test for compound VI against HepG-2 cells, indicated that compound VI can arrest cell cycle at G2/M phase, and can cause apoptosis at pre-G1 phase, with high apoptotic effect 18.53%. Molecular docking studies of the designed compounds confirmed the essential hydrogen bonding with CYS241 beside the hydrophobic interaction at the binding site compared to reference compounds which assisted in the prediction of the structure requirements for the detected antitumor activity.


 Please wait while we load your content...
Please wait while we load your content...