Chemoselective synthesis of multifunctional ferrocene-containing derivatives by the cross Rauhut–Currier reaction†
Abstract
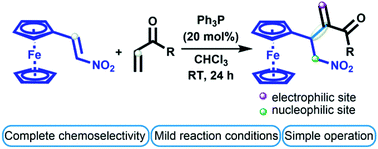
A simple protocol has been developed for the chemoselective synthesis of ferrocene-containing Rauhut–Currier adducts from 1-ferrocenyl-2-nitroethene and vinyl ketones using 20 mol% of triphenylphosphine. Multifunctional ferrocene derivatives were obtained in moderate to high yields (51–92%) by the coupling between the α-position of vinyl ketones and the β-position of the nitroalkene. The study of the Rauhut–Currier reaction under the described conditions showed that the strong electron-donating group at the β-position of nitroalkenes plays a significant role in the reaction outcome due to prevention of polymerization and stabilization of the zwitterionic intermediate. Additionally, a preparative synthesis of 4-ferrocenyl-3-methylene-5-nitropentan-2-one was carried out and its synthetic transformations showed easy conversion to other useful building blocks.


 Please wait while we load your content...
Please wait while we load your content...