Highly efficient Ru(ii)–alkylidene based Hoveyda–Grubbs catalysts for ring-closing metathesis reactions†
Abstract
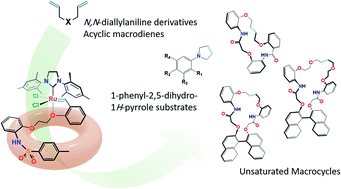
Three novel phosphine-free Ru-alkylidenes (7a–7c) have been synthesized and utilized as efficient catalysts for ring closing metathesis (RCM) reaction. Spectroscopic data, i.e. NMR and HRMS, along with single crystal X-ray diffraction analysis, were used to confirm their chemical structures. The tosylated carbenoid 7b showed the highest efficiency in cyclizing different acyclic diene substrates. RCM of various (un)substituted N,N-diallylaniline derivatives and stereoselective RCM of different macromolecular dienes were well tolerated using only a catalytic amount (0.5–2.0 mol%) of the additive catalyst (7b) as compared to the well-known Grubbs (II) and Hoveyda–Grubbs (II) catalysts.


 Please wait while we load your content...
Please wait while we load your content...