C(acyl)–C(sp2) and C(sp2)–C(sp2) Suzuki–Miyaura cross-coupling reactions using nitrile-functionalized NHC palladium complexes†
Abstract
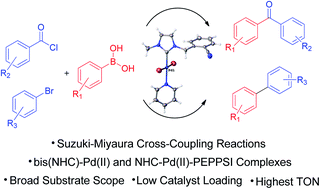
Application of N-heterocyclic carbene (NHC) palladium complexes has been successful for the modulation of C–C coupling reactions. For this purpose, a series of azolium salts (1a–f) including benzothiazolium, benzimidazolium, and imidazolium, bearing a CN-substituted benzyl moiety, and their (NHC)2PdBr2 (2a–c) and PEPPSI-type palladium (3b–f) complexes have been systematically prepared to catalyse acylative Suzuki–Miyaura coupling reaction of acyl chlorides with arylboronic acids to form benzophenone derivatives in the presence of potassium carbonate as a base and to catalyse the traditional Suzuki–Miyaura coupling reaction of bromobenzene with arylboronic acids to form biaryls. All the synthesized compounds were fully characterized by Fourier Transform Infrared (FTIR), and 1H and 13C NMR spectroscopies. X-ray diffraction studies on single crystals of 3c, 3e and 3f prove the square planar geometry. Scanning Electron Microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), metal mapping analyses and thermal gravimetric analysis (TGA) were performed to get further insights into the mechanism of the Suzuki–Miyaura cross coupling reactions. Mechanistic studies have revealed that the stability and coordination of the complexes by the CN group are achieved by the removal of pyridine from the complex in catalytic cycles. The presence of the CN group in the (NHC)Pd complexes significantly increased the catalytic activities for both reactions.


 Please wait while we load your content...
Please wait while we load your content...