Conversion of N-acyl amidines to amidoximes: a convenient synthetic approach to molnupiravir (EIDD-2801) from ribose†
Abstract
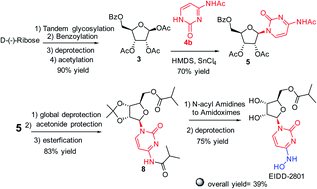
An efficient method is described for the preparation of molnupiravir (EIDD-2801) an antiviral agent via regioselective conversion of an N-acyl-nucleoside intermediate, generated through stereo and regioselective glycosylation of protected ribose and N4-acetyl cytosine, to an amidoxime. This method avoids use of expensive starting materials, enzymes, complex reagents, and cumbersome purification procedures.
- This article is part of the themed collection: 2021 Outstanding Student Paper Awards


 Please wait while we load your content...
Please wait while we load your content...