Enantio- and regioselective asymmetric allylic substitution using a chiral aminophosphinite ruthenium complex: an experimental and theoretical investigation†
Abstract
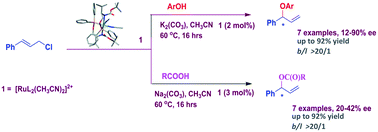
The design and synthesis of a new chiral aminophosphinite-ligated ruthenium complex is described. The ruthenium complex, [Ru(AMP)2(CH3CN)2][BPh4]2 {AMP = (S)-tert-butyl 1-(diphenylphosphinooxy)-3-methylbutan-2-ylcarbamate}, has been found to catalyze nucleophilic addition of phenol and carboxylic acid to allyl chloride in a highly regioselective fashion with enantiomeric excess ranging from 12 to 90.


 Please wait while we load your content...
Please wait while we load your content...