BSA–MnO2–SAL multifunctional nanoparticle-mediated M1 macrophages polarization for glioblastoma therapy
Abstract
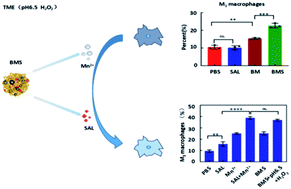
Glioblastoma (GBM) is a type of brain tumour with a very high fatality rate. Owing to the presence of the blood–brain barrier (BBB), it is difficult for drugs to reach the tumour site; thus, there has been little progress in GBM chemotherapeutics. Furthermore, the malignant growth of tumours largely depends on the tumour microenvironment. GBM is especially prevalent in slightly acidic, hydrogen peroxide (H2O2)-rich, hypoxic, and immunosuppressive microenvironments. Tumour-supporting macrophages (M2 macrophages) are a type of immune cell that promote tumour growth. Therefore, targeting M2 macrophages and repolarizing them into tumour-suppressor macrophages (M1 macrophages) are important strategies for GBM treatment. Salinomycin (SAL) is an anti-tumour drug that can improve the tumour immune microenvironment. Interestingly, we found that SAL promoted the expression of M1 macrophages in vitro, but its ability was limited in vivo because of the presence of the BBB. In this study, we combined SAL and MnO2 to design bovine serum albumin–MnO2–SAL (BMS), a nanoparticle that responds to acidic and H2O2-rich microenvironments. Our experimental results showed that BMS reduced GBM growth efficiency and had the ability to penetrate the BBB. It also enhanced the repolarization ability of SAL owing to the production of Mn2+ after decomposition, which could be applied in Magnetic Resonance Imaging (MRI). This study demonstrated that the multifunctional nanoparticle BMS is of great significance in inhibiting orthotopic GBM growth and improving immunosuppressive microenvironments.


 Please wait while we load your content...
Please wait while we load your content...