Solution-phase synthesis of oligodeoxyribonucleotides using the H-phosphonate method with N-unprotected 5′-phosphite monomers†
Abstract
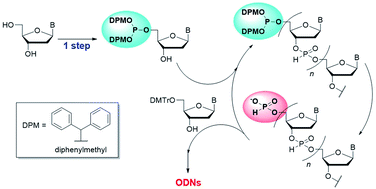
Recent advances in nucleic acid therapeutics increase the requirements for developing efficient methods for the chemical synthesis of oligodeoxyribonucleotides (ODNs). In this study, we report a new approach for the solution-phase synthesis of ODNs using the H-phosphonate method with N-unprotected 5′-phosphite monomers. The 5′-phosphite monomers are synthesized in a single step from unprotected 2′-deoxyribonucleosides using 5′-O-selective phosphitylation and can be applied to the synthetic cycle of the H-phosphonate method. We synthesized four kinds of 5′-phosphite monomers and then optimized the conditions for the condensation between the 3′-hydroxy groups of the 5′-phosphite monomers and the H-phosphonate monoesters. As a result of various investigations, solution-phase synthesis of trithymidine diphosphate (TTT) and tetramers containing four kinds of nucleobases was achieved according to the procedure consisting of repeated condensation, deprotection, and purification using simple extraction or precipitation.


 Please wait while we load your content...
Please wait while we load your content...